Mesomeric betaine – N-heterocyclic carbene interconversions of 1,2,4-triazolium-phenolates. Sulfur, selenium, and borane adduct formation†
Abstract
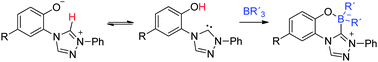
The conjugated mesomeric betaines 2-(1-phenyl-4H-1,2,4-triazolium-4-yl)phenolates are masked N-heterocyclic carbenes of 1,2,4-triazole which can be trapped as thiones and selenones. Reaction with triethylborane and triphenylborane resulted in the formation of first representatives of a new zwitterionic heterocyclic ring system, benzo[e]1,2,4-triazolo[3,4-c][1,4,2]oxazaborinium-4-ide, as a formal trapping product of an anionic N-heterocyclic carbene.

 Please wait while we load your content...
Please wait while we load your content...