The structure of a ferrous heme-nitro species in the binuclear heme a3/CuB center of ba3-cytochrome c oxidase as determined by resonance Raman spectroscopy†
Abstract
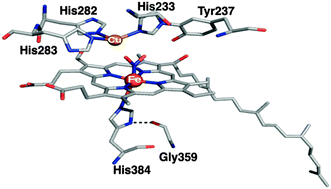
Members of the cytochrome c oxidase family exhibit nitrite reductase activity. In this work, we have characterized a ferrous heme a3-nitro species in ba3-oxidase by resonance Raman spectroscopy. This provides the first evidence for the structure of a nitrite-bound species in the binuclear heme/copper center of cytochrome c oxidases.

 Please wait while we load your content...
Please wait while we load your content...