Palladium(ii)-catalyzed meta-selective direct arylation of O-β-naphthyl carbamate†
Abstract
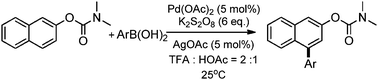
Selective meta-arylation of O-β-naphthyl carbamate has been accomplished using Pd(OAc)2 as a catalyst precursor and K2S2O8 with AgOAc as the oxidant. A range of aryl boronic acids could be introduced in moderate to good yields. Mechanistic investigation shows that the carbamate substituent has a unique effect and the reaction undergoes an ortho-carbometallation/meta-direct arylation process.

 Please wait while we load your content...
Please wait while we load your content...