Mn(OAc)3-mediated phosphonation–lactonization of alkenoic acids: synthesis of phosphono-γ-butyrolactones†‡
Abstract
A new, general method for the synthesis of phosphono-γ-butyrolactones has been achieved through Mn(OAc)3-mediated radical oxidative phosphonation and lactonization of alkenoic acids with H-phosphonates and H-phosphine oxide. Mn(OAc)3 can be readily prepared from Mn(OAc)2 in the laboratory. This transformation allows the direct formation of a P–C bond and the construction of a lactone ring in one reaction.
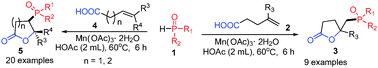

 Please wait while we load your content...
Please wait while we load your content...