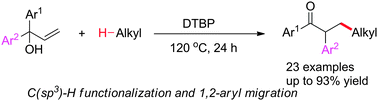
Metal-free oxidative C(sp3)–H bond functionalization of alkanes and alkylation-initiated radical 1,2-aryl migration in α,α-diaryl allylic alcohols†
Abstract
An oxidative C(sp3)–H bond functionalization of alkanes and alkylation-initiated radical 1,2-aryl migration in α,α-diaryl allylic alcohols using di-tert-butyl peroxide (DTBP) as the oxidant was established, which tolerates a wide range of simple alkane substrates and α,α-diaryl allylic alcohols for direct preparation of α-aryl-β-alkylated carbonyl ketones.

 Please wait while we load your content...
Please wait while we load your content...