Indium(iii) chloride catalyzed highly diastereoselective domino synthesis of indenodithiepines and indenodithiocines†
Abstract
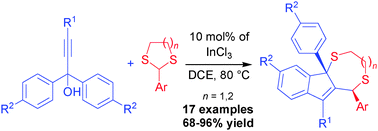
An atom-economical diastereoselective synthesis of indenodithiepines and indenodithiocines has been developed via a domino reaction of propargylic alcohols and dithioacetals in the presence of InCl3 as a catalyst. A range of functionalized dithiepines and dithiocines, fused to the indene ring, were obtained in good to excellent yields under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...