Orthogonal selectivity with cinnamic acids in 3-substituted benzofuran synthesis through C–H olefination of phenols†
Abstract
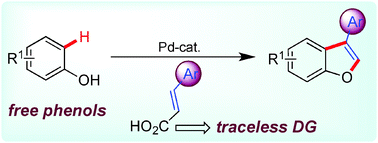
A palladium catalyzed intermolecular annulation of cinnamic acids and phenols has been achieved for the selective synthesis of 3-substituted benzofurans. Isotope labeling, competition experiments, kinetic studies, and intermediate trapping have supported a sequence of C–C bond formation and decarboxylation followed by the C–O cyclization pathway.
- This article is part of the themed collection: 2015 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...