Stereoselective synthesis of ε-lactones or spiro-heterocycles through NHC-catalyzed annulation: divergent reactivity by catalyst control†
Abstract
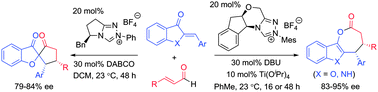
NHC-catalyzed divergent annulation of enals with heterocyclic enones was developed to produce benzofuran/indole-containing ε-lactones or spiro-heterocycles in a highly diastereo- and enantioselective fashion. The chemo-selectivity controlled by the chiral catalyst backbone is particularly noteworthy.

 Please wait while we load your content...
Please wait while we load your content...