A new method for peptide synthesis in the N→C direction: amide assembly through silver-promoted reaction of thioamides†
Abstract
The Ag(I)-promoted coupling of amino acids and peptides with amino ester thioamides generates peptide imides without epimerisation. The peptide imides undergo regioselective hydrolysis under mild conditions to generate native peptides. This method was employed to prepare the pentapeptide thymopentin in the N→C direction, in high yield and purity.
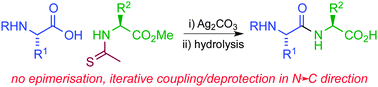

 Please wait while we load your content...
Please wait while we load your content...