Copper-catalyzed enantioselective 1,4-addition of alkyl groups to N-sulfonyl imines†
Abstract
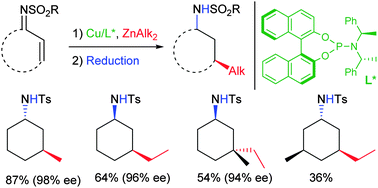
In copper(I)/phosphoramidite-catalyzed asymmetric 1,4-additions of dialkylzinc, N-sulfonyl imines are more reactive and furnish higher enantiomeric excesses than the respective cycloalk-2-enones. This enables formation of a quaternary stereocenter as well as a cis-selective addition to an imine derived from 5-methylcyclohex-2-enone. The 1,4-adducts can be transformed in stereodivergent reductions yielding cis- or trans-3-alkylcycloalkyl amides.

 Please wait while we load your content...
Please wait while we load your content...