Highly diastereo- and enantioselective copper-catalyzed propargylic alkylation of acyclic ketone enamines for the construction of two vicinal stereocenters†
Abstract
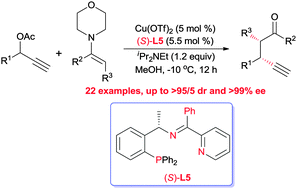
The first highly diastereo- and enantioselective propargylic alkylation of acyclic ketone enamines to form vicinal tertiary stereocenters has been reported by employing copper catalysis in combination with a bulky and structurally rigid tridentate ketimine P,N,N-ligand.

 Please wait while we load your content...
Please wait while we load your content...