Organic mixed valency in quadruple hydrogen-bonded triarylamine dimers bearing ureido pyrimidinedione moieties†
Abstract
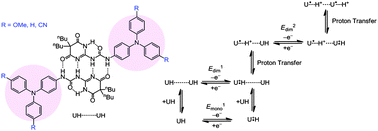
Quadruple hydrogen-bonding interactions stabilized the mixed-valence states of dimers of triarylamine derivatives bearing an ureido pyrimidinedione moiety without any electronic coupling. This study represents the first example of proton-coupled “organic” mixed valency in solution with different substituents being used to control the stability.

 Please wait while we load your content...
Please wait while we load your content...