Ruthenium-catalyzed highly regio- and stereoselective hydroarylation of aromatic sulfoxides with alkynes via C–H bond activation†
Abstract
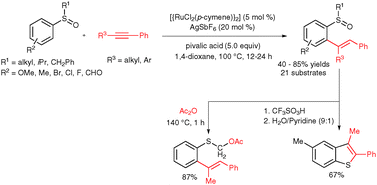
Chelation-assisted alkenylation at the ortho C–H bond of aromatic sulfoxides with alkynes in the presence of a ruthenium catalyst, AgSbF6 and pivalic acid yielding trisubstituted alkenes in good to excellent yields in a highly regio- and stereoselective manner via a deprotonation metalation pathway is described. Later, ortho-alkenylated aromatic sulfoxides were converted into α-acyloxy-thioether and a 2,3-disubstituted benzothiophene derivative.


 Please wait while we load your content...
Please wait while we load your content...