Conformationally switchable non-cyclic tetrapyrrole receptors: synthesis of tetrakis(1H-pyrrole-2-carbaldehyde) derivatives and their anion binding properties†
Abstract
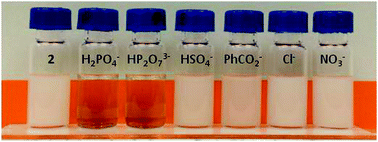
A series of tetrakis(1H-pyrrole-2-carbaldehyde) receptors (2, 4, and 6) were synthesized in two steps from commercially available starting materials. The anion binding properties of these receptors can be tuned through electronic switching to stabilize a conformation displaying high affinity for the dihydrogenphosphate and pyrophosphate anions (as the tetrabutylammonium salts) in chloroform.

 Please wait while we load your content...
Please wait while we load your content...