Copper-mediated intramolecular oxidative C–H/N–H cross-coupling of α-alkenoyl ketene N,S-acetals to synthesize pyrrolone derivatives†
Abstract
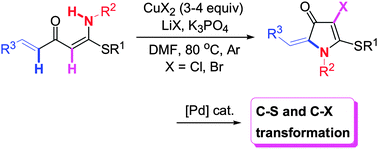
CuCl2 and CuBr2-mediated intramolecular oxidative C–H/N–H cross-coupling/halogenation of β-thioalkyl-substituted α-alkenoyl ketene N,S-acetals occurred efficiently, affording 4-halo-5-thioalkyl-3-pyrrolones. Tunable C–S and C–halo bond transformations of the resultant pyrrolone derivatives led to highly functionalized N-heterocyclic compounds.

 Please wait while we load your content...
Please wait while we load your content...