Diastereoselective synthesis of α-(aminomethyl)-γ-butyrolactones via a catalyst-free aminolactonization†
Abstract
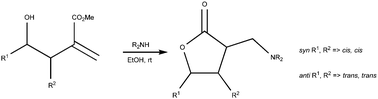
An auto-catalytic domino reaction, presumably involving an aza-Michael reaction, proton transfer, and lactonization, furnishing α-(aminomethyl)-γ-butyrolactones in near quantitative yields and excellent diastereoselectivity is described.
 Please wait while we load your content...
Please wait while we load your content...