A highly selective sulfinate ester probe for thiol bioimaging†
Abstract
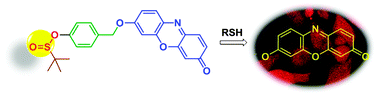
We describe here hitherto unexplored chemistry of the sulfinate ester functional group as being highly selective towards nucleophilic substitution by thiols at physiological pH. Using this cleavable trigger, an optical thiol probe that is suitable for thiol bioimaging has been developed.

 Please wait while we load your content...
Please wait while we load your content...