A facile access to a novel NHC-stabilized silyliumylidene ion and C–H activation of phenylacetylene†
Abstract
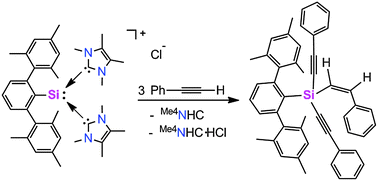
Taking advantage of two N-heterocyclic carbenes (NHCs), novel silyliumylidene ions 1a and 1b are prepared by a facile one-pot reaction of the corresponding dichlorosilanes with three equivalents of NHCs. For the first time, a C–H insertion reaction of phenylacetylene by a novel silyliumylidene ion is reported. The treatment of m-terphenyl substituted silyliumylidene ion 1a with three equivalents of phenylacetylene results in the formation of m-terphenyl substituted 1-alkenyl-1,1-dialkynylsilane 2.

 Please wait while we load your content...
Please wait while we load your content...