Mild and metal-free oxy- and amino-fluorination for the synthesis of fluorinated heterocycles†
Abstract
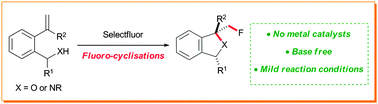
A mild intramolecular fluoro-cyclisation reaction of benzylic alcohols and amines has been developed. This strategy uses commercially available Selectfluor to trigger electrophilic cyclisations to afford fluorinated heterocycles containing 1,3-disubstitution. The dual role of the reagent as a fluorine source and a base is shown to be crucial for reactivity.

 Please wait while we load your content...
Please wait while we load your content...