Dehydromethylation of alkali metal salts of the utility amide 2,2,6,6-tetramethylpiperidide (TMP)†
Abstract
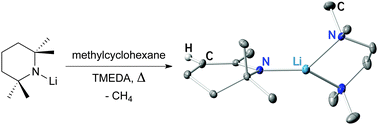
A general thermolysis reaction for the transformation of Group 1 TMP compounds (LiTMP, NaTMP, KTMP) to 1-aza-allylic TTHP derivatives is reported. TMEDA accelerates the reaction and produces the crystalline complexes [(TMEDA)LiTTHP] and [(TMEDA·NaTTHP)2]. Methane elimination during the transformational process was confirmed via TVA coupled to MS.

 Please wait while we load your content...
Please wait while we load your content...