Organocatalysed multicomponent synthesis of pyrazolidinones: Meldrum's acid approach†
Abstract
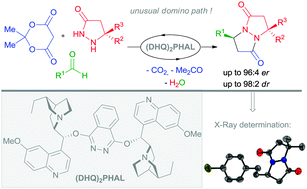
We discovered a novel organocatalysed multicomponent domino Knoevenagel-aza-Michael-cyclocondensation reaction leading to an unprecedented straightforward synthesis of 1,5-diazabicyclo[3.3.0]octane-2,6-diones. The specific capability of the (DHQ)2PHAL organocatalyst in this process was also highlighted to eventually furnish the corresponding bicyclopyrazolidinones with up to 96 : 4 er.

 Please wait while we load your content...
Please wait while we load your content...