Abstract
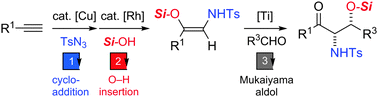
A new procedure for the stereoselective synthesis of syn α-amino β-oxy ketones is reported. It consists of two steps; in the first step, α-amino silyl enol ethers having a (Z) geometry are prepared from 1-alkynes via 1-sulfonyl-1,2,3-triazoles. In the second step, the silyl enol ethers undergo the TiCl4-mediated Mukaiyama aldol reaction with aldehydes to produce α-amino β-oxy ketones with excellent syn-selectivity.

 Please wait while we load your content...
Please wait while we load your content...