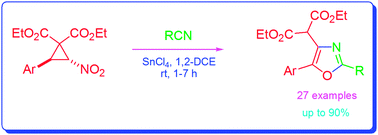
Synthesis of 2,4,5-trisubstituted oxazoles through tin(iv) chloride-mediated reaction of trans-2-aryl-3-nitro-cyclopropane-1,1-dicarboxylates with nitriles†
Abstract
trans-2-Aryl-3-nitro-cyclopropane-1,1-dicarboxylates when reacted with nitriles in the presence of tin(IV) chloride afford 2,4,5-trisubstituted oxazoles in good to excellent yields. The reactions take place through in situ generation of aroylmethylidene malonates from the cyclopropanes, followed by conjugate addition of nitriles to the malonates to form nitrilium ion intermediates and subsequent cyclisation.

 Please wait while we load your content...
Please wait while we load your content...