Covalency of hydrogen bonds in solids revisited†
Abstract
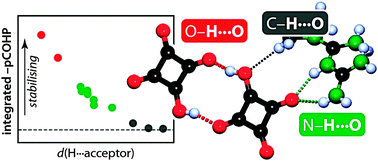
The covalent nature of short hydrogen bonds has been under debate for long. Here we show that the crystal orbital Hamilton population (COHP) bonding indicator gives new, complementary evidence of covalent hydrogen⋯acceptor interactions in the molecular solid state.

 Please wait while we load your content...
Please wait while we load your content...