Stepwise π-extension of meso-alkylidenyl porphyrins through sequential 1,3-dipolar cycloaddition and redox reactions†
Abstract
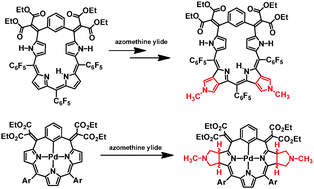
Several regioselectively π-extended, pyrrole fused porphyrinoids have been synthesized by the 1,3-dipolar cycloaddition of meso-alkylidene-(benzi)porphyrins. Pd(II) complexes gave oxidation resistant, bis-pyrrole fused adducts. The repeated 1,3-dipolar cycloaddition followed by oxidation–reduction of pentaphyrin analogs afforded π-extended porphyrin analogs.

 Please wait while we load your content...
Please wait while we load your content...