Palladium/sulfoxide–phosphine-catalyzed highly enantioselective allylic etherification and amination†
Abstract
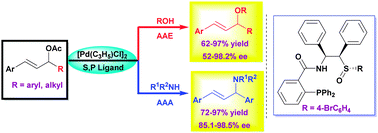
The Pd/sulfoxide–phosphine-catalyzed highly enantioselective allylic etherification and amination with a wide range of O- and N-nucleophiles have been developed (up to 97% yield, 98.5% ee). The products can also be conveniently transformed into biologically active chiral heterocycles.

 Please wait while we load your content...
Please wait while we load your content...