Diastereoselective synthesis of novel aza-diketopiperazines via a domino cyclohydrocarbonylation/addition process†
Abstract
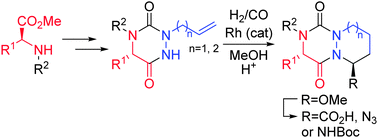
Herein, we report an unprecedented, short and diastereo-selective synthesis of newly reported aza-diketopiperazine (aza-DKP) scaffolds starting from amino acids. The strategy is based on a Rh(I)-catalyzed hydroformylative cyclohydrocarbonylation of allyl-substituted aza-DKP, followed by a diastereoselective functionalization of the platform. This methodology allows the synthesis of novel bicyclic and tricyclic aza-DKP scaffolds incorporating six- or seven-membered rings, with potential applications in medicinal chemistry.

 Please wait while we load your content...
Please wait while we load your content...