Iron or boron-catalyzed C–H arylthiation of substituted phenols at room temperature†
Abstract
A simple, efficient and environmentally friendly method for iron or boron-catalyzed C–H arylthiation of substituted phenols at room temperature has been developed, and the corresponding diaryl sulfides were prepared in good to excellent yields. The protocol uses readily available 1-(substituted phenylthio)pyrrolidine-2,5-diones as the arylthiation reagents and inexpensive and environmentally friendly FeCl3 or BF3·OEt2 as the catalyst, moreover no ligands, additives or extrusion of air are required, and the reactions can be performed successfully at room temperature.
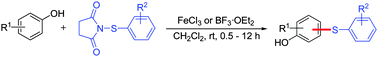

 Please wait while we load your content...
Please wait while we load your content...