Abstract
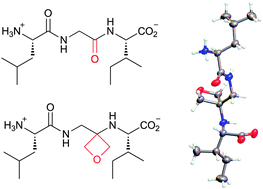
A new class of peptidomimetic is reported in which one of the amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds of the peptide backbone is replaced by an oxetane ring. They are synthesised by conjugate addition of various α-amino esters to a 3-(nitromethylene)oxetane, reduction of the nitro group and further coupling with N–Z protected amino acids to grow the peptide chain. Structural insights are provided by X-ray diffraction and molecular dynamics simulations.
O bonds of the peptide backbone is replaced by an oxetane ring. They are synthesised by conjugate addition of various α-amino esters to a 3-(nitromethylene)oxetane, reduction of the nitro group and further coupling with N–Z protected amino acids to grow the peptide chain. Structural insights are provided by X-ray diffraction and molecular dynamics simulations.
- This article is part of the themed collection: In Celebration of Richard Taylor’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...