A metal-free dyotropic-like rearrangement of 2-oxa allylic alcohols in the presence of organoboronic acids†
Abstract
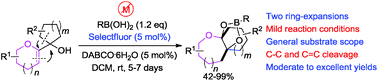
The first example of a dyotropic-like rearrangement of 2-oxa allylic alcohols in the presence of catalytic amounts of Selectfluor and DABCO was reported, which provides a facile access to organoboronates. This reaction represents an unprecedented dyotropic rearrangement consisting of cleavage of two vicinal bonds (one C–C bond and one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond).
C bond).

 Please wait while we load your content...
Please wait while we load your content...