DABCO-catalyzed ring opening of activated cyclopropanes and recyclization leading to γ-lactams with an all-carbon quaternary center†
Abstract
A novel and efficient method for the construction of γ-lactams with an all-carbon quaternary center is developed via a DABCO-catalyzed reaction of EWG-activated cyclopropanecarboxamides and electron-deficient alkenes. The process involves sequential ring-opening of activated cyclopropanes, intermolecular Michael addition and intramolecular aza-cyclization.
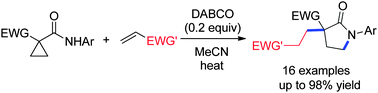

 Please wait while we load your content...
Please wait while we load your content...