The benzoyl peroxide-promoted functionalization of simple alkanes with 2-aryl phenyl isonitrile†
Abstract
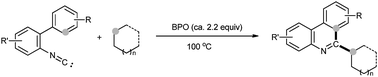
The benzoyl peroxide (BPO)-promoted phenanthridinylation of simple alkanes with isonitrile is developed via C(sp3)–H and C(sp2)–H bond cleavage. This procedure is featured by dual C–C bond formation proceeding with the addition of an alkyl radical to isonitrile followed by radical aromatic cyclization.

 Please wait while we load your content...
Please wait while we load your content...