Synthesis and ligand-based reduction chemistry of boron difluoride complexes with redox-active formazanate ligands†
Abstract
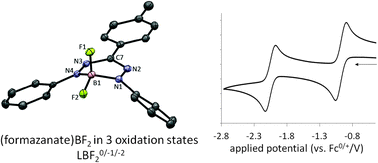
Mono(formazanate) boron difluoride complexes (LBF2), which show remarkably facile and reversible ligand-based redox-chemistry, were synthesized by transmetallation of bis(formazanate) zinc complexes with boron trifluoride. The one-electron reduction product [LBF2]−[Cp2Co]+ and a key intermediate for the transmetallation reaction, the six-coordinate zinc complex (L(BF3))2Zn were isolated and fully characterized.
- This article is part of the themed collection: Non-Innocent Ligands

 Please wait while we load your content...
Please wait while we load your content...