From π-expanded coumarins to π-expanded pentacenes†
Abstract
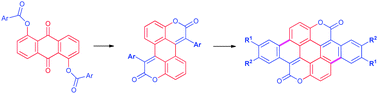
The synthesis of two novel types of π-expanded coumarins has been developed. Modified Knoevenagel bis-condensation afforded 3,9-dioxa-perylene-2,8-diones. Subsequent oxidative aromatic coupling or light driven electrocyclization reaction led to dibenzo-1,7-dioxacoronene-2,8-dione. Unparalleled synthetic simplicity, straightforward purification and superb optical properties have the potential to bring these perylene and coronene analogs towards various applications.

 Please wait while we load your content...
Please wait while we load your content...