Pd-Catalyzed C–H activation/oxidative cyclization of acetanilide with norbornene: concise access to functionalized indolines†
Abstract
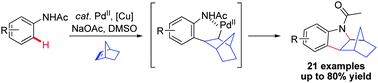
An efficient Pd-catalyzed oxidative cyclization reaction for the synthesis of functionalized indolines by direct C–H activation of acetanilide has been developed. The norbornylpalladium species formed via direct ortho C–H activation of acetanilides is supposed to be a key intermediate in this transformation.

 Please wait while we load your content...
Please wait while we load your content...