Reversible mechanochromism and enhanced AIE in tetraphenylethene substituted phenanthroimidazoles†
Abstract
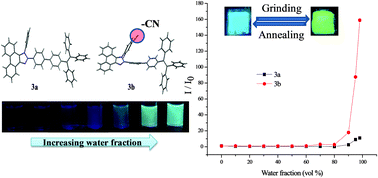
Tetraphenylethene (TPE) substituted phenanthroimidazoles 3a and 3b were designed and synthesized by the Suzuki cross-coupling reaction. They show reversible mechanochromic behavior with contrast colors between sky-blue and yellow green. The powder XRD studies show that destruction of a crystalline state into an amorphous state is responsible for mechanochromism. Hydrogen bonding interaction of a cyano-group in 3b results in enhanced AIE and improved thermal stability.
- This article is part of the themed collection: 20th Anniversary of the CRSI: Celebrating Indian Chemistry in ChemComm

 Please wait while we load your content...
Please wait while we load your content...