Total synthesis of securinega alkaloids (−)-norsecurinine, (−)-niruroidine and (−)-flueggine A†
Abstract
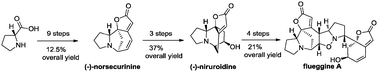
A consecutive synthetic strategy was developed toward the total synthesis of securinega alkaloids. (−)-Norsecurinine was concisely assembled by addition of a methoxyallene to a ketone for efficient side-chain installation. Ring-closing metathesis was also utilized as a key step. The first total synthesis of (−)-niruroidine was achieved from (−)-norsecurinine in three steps, while the route to (−)-flueggine A featured a 1,3-dipolar cycloaddition to forge the core structure.

 Please wait while we load your content...
Please wait while we load your content...