Pd-catalyzed highly regio-, diastereo-, and enantioselective allylic alkylation of α-fluorophosphonates†
Abstract
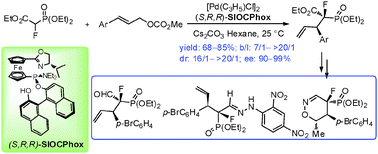
Highly efficient Pd-catalyzed asymmetric allylic alkylation reaction of ethyl-2-fluoro-2-(diethoxyphosphoryl)acetate with monosubstituted allylic substrates has been developed, affording corresponding α-fluorophosphonates with two chiral centers in high regio-, diastereo- and enantio-selectivities. The usefulness of the products in organic synthesis has been demonstrated.

 Please wait while we load your content...
Please wait while we load your content...