Organocatalytic enantioselective and (Z)-selective allylation of 3-indolylmethanol via hydrogen-bond activation†
Abstract
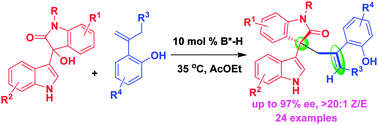
An organocatalytic asymmetric allylation of 3-indolylmethanol has been established via hydrogen-bond activating mode, which directly assembles isatin-derived 3-indolylmethanols and o-hydroxystyrenes into chiral allyl-substituted oxindoles with one all-carbon quaternary stereogenic center and one newly formed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in excellent enantioselectivity and (Z)-selectivity (up to 97% ee, >20 : 1 Z/E ratio). This transformation provides an efficient strategy for asymmetric C3-functionalization of indoles and allylation of 3-indolylmethanols with precise control of the stereoselectivity in the formation of C–C and C
C bond in excellent enantioselectivity and (Z)-selectivity (up to 97% ee, >20 : 1 Z/E ratio). This transformation provides an efficient strategy for asymmetric C3-functionalization of indoles and allylation of 3-indolylmethanols with precise control of the stereoselectivity in the formation of C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds.
C bonds.

 Please wait while we load your content...
Please wait while we load your content...