Dual-function Pd/NHC catalysis: tandem allylation–isomerization–conjugate addition that allows access to pyrroles, thiophenes and furans†
Abstract
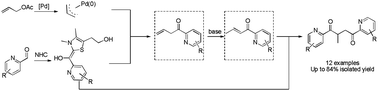
An efficient coupling reaction of allyl acetate with (O-azaaryl)carboxaldehyde by Pd–NHC dual catalysis has been developed. This reaction proceeds via direct coordination between the ortho nitrogen atom in the heterocycle and Pd(0). This dual catalysis is achieved under mild conditions to give 1,4-diones as products with up to 90% yield.

 Please wait while we load your content...
Please wait while we load your content...