Rhodium(iii)-catalyzed C2-selective carbenoid functionalization and subsequent C7-alkenylation of indoles†
Abstract
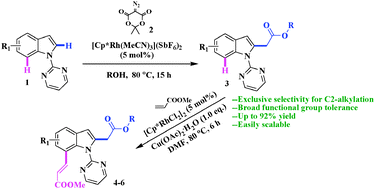
Here a new, mild and versatile method for efficient synthesis of a diverse range of 2-acetate substituted indoles via Rh(III)-catalyzed and alcohol-mediated C2-selective carbenoid insertion functionalization of indoles by α-diazotized Meldrum's acid has been developed. Furthermore, for the first time, a Rh(III)/Cu(II)-catalyzed direct C7-alkenylation of such functionalized products has also been demonstrated.

 Please wait while we load your content...
Please wait while we load your content...