n-Bu4NI/TBHP-catalyzed direct amination of allylic and benzylic C(sp3)–H with anilines under metal-free conditions†
Abstract
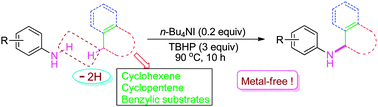
A novel and efficient n-Bu4NI/TBHP-catalyzed direct amination of allylic and benzylic C(sp3)–H with anilines to form N-substituted anilines under metal-free conditions has been developed.

 Please wait while we load your content...
Please wait while we load your content...