The carbomethylation of arylacrylamides leading to 3-ethyl-3-substituted indolin-2-one by cascade radical addition/cyclization†
Abstract
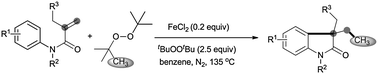
An FeCl2-promoted carbomethylation of arylacrylamides by di-tert-butyl peroxide (DTBP) is achieved, leading to 3-ethyl-3-substituted indolin-2-one in high yield. The reaction tolerates a series of functional groups, such as cyano, nitro, ethyloxy carbonyl, bromo, chloro, and trifluoromethyl groups. The radical methylation and arylation of the alkenyl group are involved in this reaction.

 Please wait while we load your content...
Please wait while we load your content...