tert-Butyl peroxybenzoate (TBPB)-mediated 2-isocyanobiaryl insertion with 1,4-dioxane: efficient synthesis of 6-alkyl phenanthridines via C(sp3)–H/C(sp2)–H bond functionalization†
Abstract
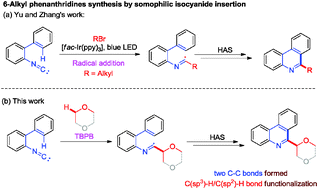
An efficient method for the construction of 6-alkyl phenanthridines by tert-butyl peroxybenzoate (TBPB)-mediated 2-isocyanobiaryl insertion with 1,4-dioxane was established. Two new C–C bonds were formed in this reaction via a sequential C(sp3)–H/C(sp2)–H bond functionalization under metal-free conditions.

 Please wait while we load your content...
Please wait while we load your content...