Iron-catalyzed carbonylative Suzuki reactions under atmospheric pressure of carbon monoxide†
Abstract
The first highly effective iron-catalyzed carbonylative Suzuki reaction has been developed. Substrates with electron-donating or electron-withdrawing functionality, ortho-substitution, as well as active groups proceeded smoothly, affording desired products in high yields. This protocol is economical, environmentally benign and practical for the synthesis of biaryl ketones.
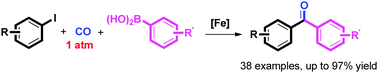

 Please wait while we load your content...
Please wait while we load your content...