MeOTf-induced carboannulation of arylnitriles and aromatic alkynes: a new metal-free strategy to construct indenones†
Abstract
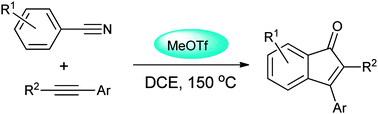
MeOTf-induced carboannulation of arylnitriles and aromatic alkynes for synthesis of indenones under metal-free conditions has been described. When ortho-substituted benzonitriles were used, indeno[1,2-c]isoquinolines were formed.

 Please wait while we load your content...
Please wait while we load your content...