Enantioselective formal α-allylation of nitroalkanes through a chiral iminophosphorane-catalyzed Michael reaction–Julia–Kocienski olefination sequence†
Abstract
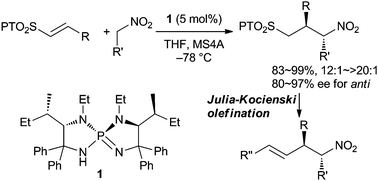
A two-step sequence for the asymmetric formal α-allylation of nitroalkanes is disclosed. This new methodology relies on the development of a highly diastereo- and enantioselective conjugate addition of nitroalkanes to vinylic 2-phenyl-1H-tetrazol-5-ylsulfones using chiral triaminoiminophosphorane as a requisite base catalyst and subsequent Julia–Kocienski olefination under kinetic conditions.

 Please wait while we load your content...
Please wait while we load your content...