Highly enantioselective synthesis of α-azido-β-hydroxy methyl ketones catalyzed by a cooperative proline–guanidinium salt system†
Abstract
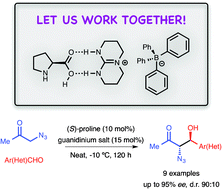
The combined activity of (S)-proline and an achiral tetraphenylborate TBD-derived guanidinium salt permits the aldol reaction between azidoacetone and aromatic, or heteroaromatic aldehydes. The α-azido-β-hydroxy methyl ketones obtained as products can be isolated in good yield, with high diastereo- and enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...