Oxygen evolution reaction electrocatalyzed on a Fenton-treated gold surface†
Abstract
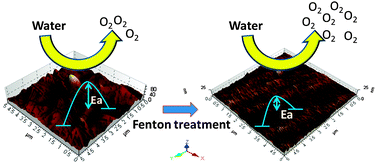
Hydroxyl radicals arising from the Fenton reagent remove metastable surface gold atoms (low coordinated high-energy surface atoms) on the Au surface, thus precluding the formation of stable oxides. The resultant smooth surface, upon hydroxyl-radical activation, electrocatalyzes oxygen evolution reaction in 0.1 M NaOH at overpotentials lowered by 190 mV @ 10 mA cm−2.

 Please wait while we load your content...
Please wait while we load your content...