The four-step total synthesis of (−)-chaetominine†
Abstract
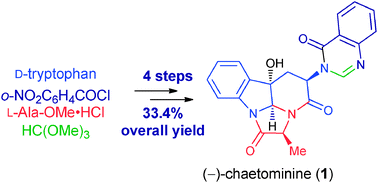
The total synthesis of the alkaloid (−)-chaetominine (1) has been achieved in four steps with an overall yield of 33.4%. Key features of our strategy include a one-pot cascade indole epoxidation – epoxide ring-opening cyclization – lactamization reaction sequence, and the use of a nitro group as a latent amino group for the one-pot construction of the quinazolinone ring. This constitutes a step economical, redox economical and protecting group-free total synthesis.

 Please wait while we load your content...
Please wait while we load your content...